Gems and Precious Stones
Gems and Precious Stones
A Tabular Arrangement of Their Characteristics and Localities
with some
Tests and Literature on the Subject
Published by The Jewelers' Circular Publishing Co., New York
The text that follows was issued in 1906.
The text begins with a table of Characteristics and Localities of the Principal Precious Stones (PDF). The table spans the width of facing pages, so it's suggested that you download the PDF to view in Adobe Reader.
Characteristics and Localities of the Principal Precious Stones
The text begins with a table of Characteristics and Localities of the Principal Precious Stones (PDF).
Tests for Precious Stones
by
E. Hopkins.
From
The Jewelers' Circular—Weekly.
September 12, 1906.
THERE is an old saying that "All the good die young," but the modern version in the jewelry trade should be, that all the good come to life when old, speaking geologically of sparkling gems. The list accompanying this has been compiled with the idea of giving in a concrete form a ready system of reference of some of the tests for precious stones, and is a reduction of a larger one mounted on card and measuring 19½ x 15 inches.
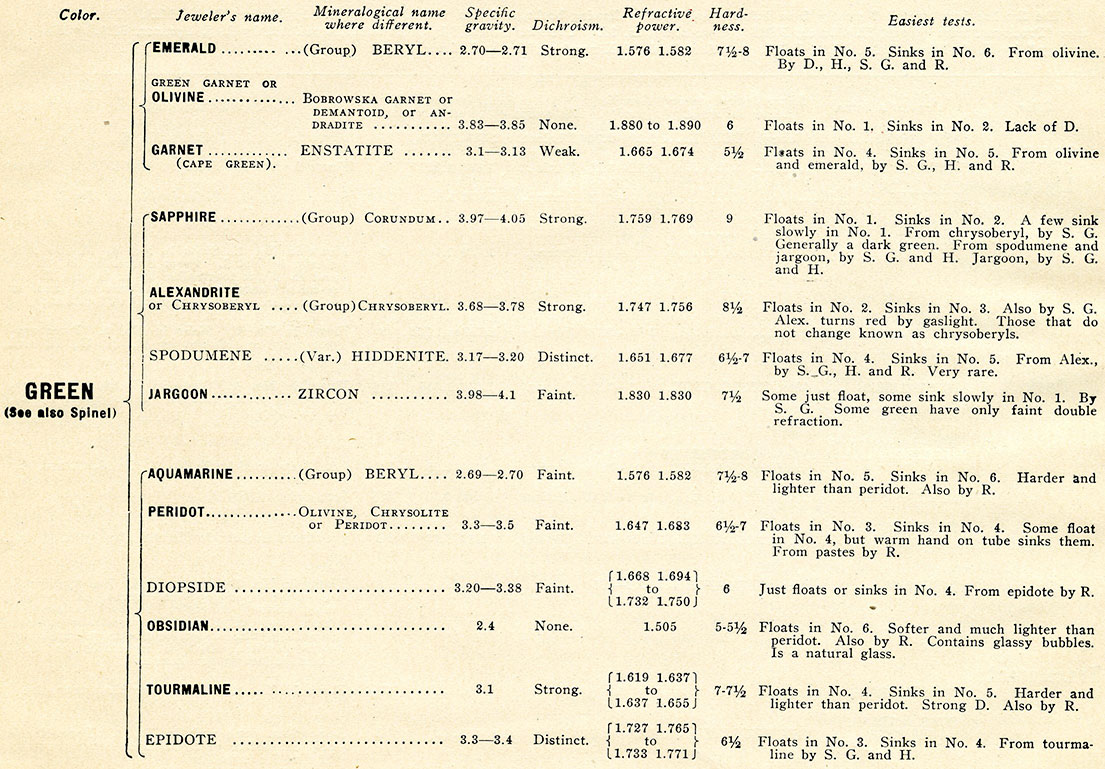
At first sight, perhaps, the list appears to contain a formidable number of names and details, but upon examination it will be found to be simple enough. Color, being the most obvious character of a stone, is taken as an index. Thus, if we have, say, a pink stone, it is only necessary to examine the five species in the list possessing that color. Many names are necessarily repeated on account of the numerous varieties of color. For instance, tourmaline is mentioned no fewer than seven and sapphire six times, and so on. A few very rare colors are not included, but the tests in such cases are, of course, precisely the same.
Any doubtful specimen which would be encountered in the ordinary course of business will always be found under one of the headings of the list. Considerable confusion might be caused by reference to the textbooks, since not only do the trade and mineralogists often give different names to the same species or variety, but they even, in one case at least, apply a particular name to entirely distinct species. Thus peridot is termed by mineralogists olivine or chrysolite; and, on the other hand, olivine (green garnet) is known to them as andradite or demantoid. It is hoped that the criteria accompanying this article will remove any doubt that may arise.
The tests, and apparatus required for them, are as follows: (1) Dichroism, (2) Density or Specific Gravity, (3) Hardness; and (4) Refractive Power. By such means all gems can be discriminated. Of course, there are other tests, but they have either restricted application or require elaborate and expensive apparatus.
To test the dichroism, we require an inexpensive little instrument about two inches long, known as the dichroscope. It is very useful for many colored stones and will generally give the result in a few seconds.
The necessary apparatus for the determination of the specific gravity consists of three or six tubes containing liquids of different densities, so that, by placing the stone to be experimented upon in one or more of them, its specific gravity can be obtained, and, by comparison with the list, can be recognized. An extra and larger tube is also supplied to take stones up to about 50 carats each.
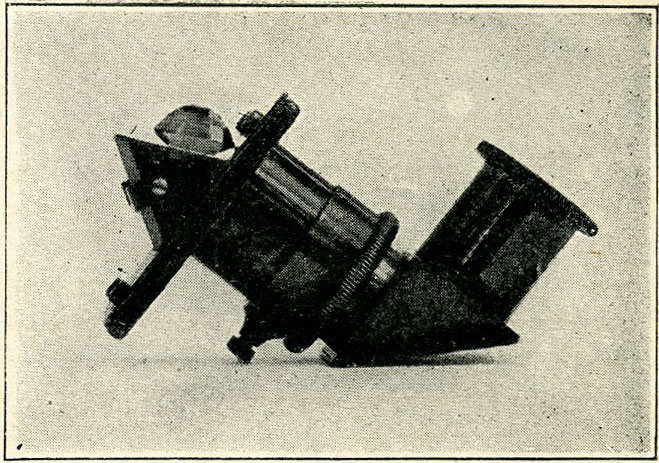
Four points of varying hardness are cemented into holders and are of use in confirming the other tests. Nos. 1, 2, 3 are supplied in a mahogany case as in illustration [Fig. 1].
Fig. 1. Showing Case and Contents.
The refractometer recently introduced by G. F. Herbert Smith, M.A., measures on a photographic scale an equivalent to the refractive index, if not exceeding 1.76, and so, as will be seen, covers a large number of precious stones; the remainder, being separated from these, can be specified to some extent negatively. There is also no calculation required with this instrument. The refractive indices correspond to the several divisions of the scale.
These four tests form a very rapid and correct means of classifying gems.
A powerful magnifying glass should always be at hand with which many characteristics can be seen that would be otherwise overlooked. The watchmakers' eyeglass with double lenses gives very good results.
It would be of great advantage if some of the technical institutes could give a course of lectures or classes—advertising them well—on "Gemmology"—a word, I believe, introduced by W. J. Lewis Abbott when he gave a series of excellent lectures some few years ago—at the same time developing the optical side a little more. I would suggest the reading of Dr. Max Bauer's book on "Precious Stones," which has been translated by L. J. Spencer, M.A., from the German edition. It is a somewhat bulky volume, but is full of interesting matter, with a great number of colored and other plates, and, I believe, is the finest of its kind that has been published. The values placed in some parts of the book are hardly in accordance with trade ideas, but otherwise the information is excellent and very clear. For a small handbook for ready reference there is nothing to equal Professor Church's "Precious Stones," either in price or information. The latter should be in the hands of all who have anything to do with the subject. The last (1905) edition has been much enlarged. A series of visits to the Museum of Practical Geology, Jermyn-street, and the British Museum (Natural History) at South Kensington, would surprise many on account of the exceedingly fine display of specimens and admirable arrangement of cases. In the latter museum this is especially so. The window cases on the left give a good introduction to the study of minerals.
Fig. 2. The Dichroscope.
The Dichroscope.
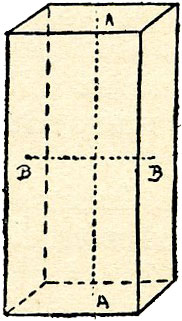
Method of Use.—In the case of those instruments having the additional fitting, the stone is slightly warmed to give a better grip in the wax, and focussed by sliding the end piece B. By means of the button A, it may be given a circular motion in one direction, and if the inner tube B is revolved, another circular movement is received at right angles, so that the stone can be examined in many directions. Failing the extra fitting, the stone is taken in the usual manner and held in front of the small square hole at one end. Light passing to the eye through the instrument is split up into two beams, and a double image of the square opening is produced. When these images, on interposition of the stone, are of different tints, the stone is said to be dichroic—two colored—(see list under D). The instrument is a good test only for stones having strong or distinct dichroism, providing they have a fair amount of color. With those in the list marked "faint" it is not very serviceable, as the comparison is often difficult to see. Those stones which in all positions show both squares the same tint are said to be "monochroic," i.e., of one color. The latter consist of only three groups, and are easily remembered: the diamond, the spinel, and the garnet. The latter include not only the ordinary garnets, but also the jeweler's jacinth and the jeweler's olivine. (The latter was formerly known as green garnet.) All the others are dichroic, some faintly so, while others are very distinct. (See list.) The darker the color, the better the result, providing light can pass through the stone. The monochroic stones all crystalize in one group, the cubic, the simplest form of which is the cube, having imaginary axes at right angles, and of equal length, AA. All non-crystaline materials are also monochroic. The remainder crystalize in various classes, and have axes of differing lengths, caused by certain molecular grouping, which gives the property of dichroism. Fig. 4 shows such axes. AA is known as the optic axis, and may be either longer or shorter than BB, but is always of different length. (For our present purpose we will ignore those having two optic axes.) Along the direction AA only the same tint will be seen, but the dichroic effect becomes more and more pronounced until BB is reached, when it is at its strongest.
Fig. 4.
Fig. 3.
In a cut stone the positions can only be found by looking at the specimen from different points of view. Some easy tests are:
| Dichroic. | Monochroic. |
| Emerald | from jeweler's oviline. |
| Sapphire & blue tourmaline | from spinel and garnet. |
| Fancy colored sapphire | from fancy colored spinel. |
The Specific Gravity Tubes.
In this test, liquids of different specific gravity are supplied in three, as illustrated (Fig. 1), and also in six glass tubes. The stones must, of course, be unmounted specimens.
Each tube is marked with the density of the contained liquid, as compared with distilled water, and numbered 1, 2, 3, 4, 5, and 6, respectively. For instance, the liquid in No. 1 is between 4.0 and 4.7, and therefore is more than four times the weight of an equal amount of distilled water. In each of the tubes are two small fragments of different minerals—"indicators"—which are to be kept in their respective tubes. With the case having three tubes of liquid, divide most of the methylene iodide into three portions, putting rather more in No. 4 than in No. 5, and a little less in No. 6. No. 4 is to be left pure. Nos. 5 and 6 are to have their densities reduced by adding benzine, drop by drop, until the indicators are in position—one at the top, and the other at the bottom of the liquid. It is very important to see that they are so, as the benzine after a time evaporates.
The liquid must also be mixed thoroughly by the glass rod, as the benzine, being lighter, is apt to remain at the top. Should, by accident, too much benzine be put in, add some more methylene iodide from No. 4, or permit the benzine to evaporate. The figures on the labels present the specific gravity of the liquid, i.e., between those of the contained fragments. The density of No. 4 can be increased, if desired, by adding iodoform, but the liquid then becomes cloudy. It should be noted that benzine is highly inflammable. Valuable turquoises should not be risked in this or other liquids, the stone being porous.
To show the use of these, let us take for example colorless stones:
| Quartz (rock crystal) | floats in No. 6 |
| Aquamarine | floats in No. 5 and sinks in No. 6 |
| Tourmaline | floats in No. 4 and sinks in No. 5 |
| Diamond | floats in No. 3 and sinks in No. 4 |
| Brazilian topaz | floats in No. 2 and sinks in No. 1 |
| Sapphire | floats in No. 1 and sinks in No. 2 |
| Jargoon | sinks in No. 1 |
In the same manner, of course, colored stones can be classified. The color in certain varieties varies slightly with the density. These are given in the list. This test is an additional one to the dichroscope; as, for example, between a blue tourmaline and a sapphire of the same color, it would be somewhat difficult positively to separate the two with the latter alone. The three tubes, Nos. 4, 5, and 6, which are available for the determination of densities not exceeding 3.3, are especially recommended as entailing scarcely any trouble, and being a very ready test. The other three are somewhat more troublesome and more expensive.
If, instead of the tubes Nos. 1, 2 and 3, the ordinary diamond scales are used (which I think preferable, for the denser varieties weighing more than one carat each), the following is the method, the fractions are converted into decimals:
| 1/2 carat = .500 | 1/16 carat = .062 |
| 1/4 carat = .250 | 1/32 carat = .031 |
| 1/8 carat = .1.25 | 1.64 carat = .015 |
| Take the weight in air of a stone, say, 2 1/4 carats | = 2.250 |
| Then the weight in water, say, 1 17/32 carats | = 1.531 ______ |
| Difference | .719 |
| Divide the weight in air by the difference and the specific gravity 3.129 will be found. |
|
| .719) | 2,250 (3.129 |
| 2.157 ______ |
|
| 0.930 719 ______ |
|
| 2110 1438 ______ |
|
| 672 |
Toluene, the specific gravity of which is .869, is better than distilled water, since its surface tension is much less, the relation being then,
| weight in air x .869 —————————————— difference between weight in air and Toluene |
= sp. gr. |
The illustration will give the general arrangement of the scales, the stone being freed from all air bubbles on the surface and suspended by a thin platinum wire in the liquid. It is better, but not absolutely necessary, to have an additional pan made. The same amount of platinum wire should be in the liquid when adjusting the balance as when weighing the stone, i.e., the pan should be lifted the same height in both cases. The usual temperature of a room is sufficient for ordinary results. Toluene should be kept in a stoppered bottle as it will otherwise rust all steel parts if kept in the same case.
Fig. 5.
The Test of Hardness.
This is useful where the hardness is distinctly different, and may be used as confirming the results obtained otherwise.
The four fragments are fixed in short holders, see illustration Fig. 1 with tubes and dichroscope, and consist of (according to Mohs' scale):
| Diamond representing | 10 |
| Sapphire representing | 9 |
| Topaz representing | 8 |
| Quartz (rock crystal) | 7 |
The diamond would naturally scratch anything softer than that represented by 10, the sapphire would mark the spinel and topaz and so on in each case. If the experiment be on a faceted stone, it should be tried on one of the bottom facets, on the corner at the back, as near as possible to the edge without chipping.
In this way a scratch will scarcely be noticed, and will not cause much injury. This test should never be applied on the front without the owner's permission. When the points become blunt, move the position by warming the cement and bringing a fresh cutting edge to the top. As there is not sufficient space in the list, it may be mentioned here, that Siberian, Auvergne (which is sometimes called by jewelers "oriental"), Uruguay and Scotch amethysts are all of the same material—quartz.
Fig. 6.
The Herbert Smith Refractometer.
This has been recently introduced, and is a very portable and convenient instrument to employ in place of the former heavy and very expensive apparatus. It is preferably used with the yellow sodium flame, while the ordinary incandescent electric light, or the yellow gas are better than daylight. In the latter the line on the scale is not quite so distinct, although giving fair readings. A drop of a certain liquid is placed between the stone and the flat surface of the hemispherical glass, the position of the edge of the dark shadow noted on the photographic scale inside, and the reading of this line compared with the graduations given on the card supplied with the instrument, which correspond to the different refractive indices. No calculation whatever is required.
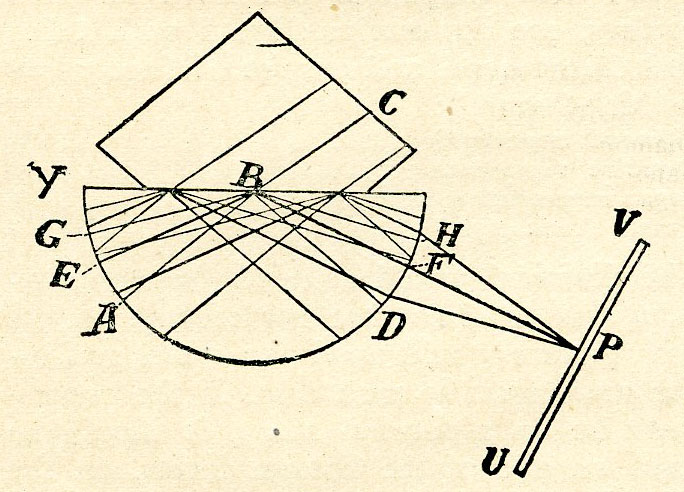
Fig. 7.
Light traversing the glass hemisphere in the direction AB (Fig. 7) is partly refracted—i.e., bent—on entering the stone along the direction BC, and partly reflected within the glass along the direction BD. If the angle ABY be reduced, it will reach a critical angle, EBY, at which the refracted rays graze the surface separating the stone and the glass. At any smaller angle—for instance, GBY—none of the light passes out into the stone, but the whole is reflected along the direction BH. Now the compensating lens (omitted for convenience in Fig. 7) is such that light reflected from the plane surface of the glass hemisphere at whatever angle comes to focus on the photographic scale UV. The critical rays, as shown in the diagram, are focussed at P. The portion of the scale towards V is illuminated by totally reflected light, and is bright by comparison with the portion towards U, which is illuminated by partially reflected light. The position of P depends on the refractive power of the stone. If the glass of the instrument be hemispherical in shape, the edge of the shadow is slightly curved. In the case of the slightly more expensive refractometer, with semi-cylindrical lens, the edge is straight.
Double refracting stones have the power of dividing the light into two rays. In certain varieties these rays are considerably apart, and there can be seen in some cases as much difference as four units in the second place of decimals, e.g., peridot. Recently there have been some pastes sold as peridots, which were in general appearance the same, and their hardness not very different, but the refractive indices (apart from the wide separation of the double rays of the peridot) are so distinct that no mistake could have been made had the refractometer been employed.
Single refracting stones and all pastes show only one line. During 1904 and 1905 many tourmalines have been found of very unusual colors. Among others, the yellows are rather interesting, and vary from an orange tint, through various shades, to the pale green varieties; also some purple-colored ones resembling spinels in tint are curious. These can be readily tested with this instrument; also the yellow spodumene can be distinguished from chrysolite, and the pink variety, kunzite, from pink topaz and tourmaline.
It is also exceedingly useful for most mounted stones; the result is not affected if the back of the stone is closed and foiled or even painted.
No damage is done to the stone experimented upon.
In these notes, the names known by jewelers have been applied.
This gives a rough outline of the principle and some uses of this invention, but the pamphlet issued with it by the maker gives much fuller particulars, and is in itself a treatise on much optical phenomena which is worth studying.
The apparatus covers a wide field in registering those transparent gems which have a refractive index not greater than 1.76 and also naturally tests those of higher refractive power by negative means.
Summary.—To sum up roughly the foregoing notes: The dichroscope easily tests those stones having strong or distinct dichroism from monochroic ones, where there is a fair amount of color.
The specific gravity tubes numbered 4, 5, and 6, are useful for both colored and colorless stones up to those having a density of 3.3.
The tubes numbered 1, 2, and 3, are useful for very small stones with greater density than 3.3. The ordinary diamond, or other delicate balance, is employed for taking the specific gravity of stones weighing more than one carat. and having a density greater than that of tube No. 4 (3.3) the liquid being either distilled water or toluene.
The Herbert Smith refractometer classifies both colored and colorless gems up to those having a refractive index of 1.76 and negatively for those beyond this, and is besides on many occasions useful for those that are mounted, whether they are foiled or not. This is one of the most rapid tests.
The points of hardness are used when the difference is considerable to confirm the other results.
After very little practice these can all be applied easily.
The subject of precious stones in general is exceedingly interesting and fascinating, and it must be a matter of great regret to the lecturers of this and kindred subjects that the opportunities held forth are so little appreciated. It would be in every way an advantage if a better general knowledge of the characteristics of gems were prevalent.
Should any of the foregoing notes not be clearly enough expressed the writer will be pleased to answer any correspondence on the subject.
The list has been compiled mostly from information in the following books, and also that kindly supplied by Herbert Smith, M.A. The volumes are: "Precious Stones," by A. H. Church, F.R.S.; paper cover, 1s. 9d. "Precious Stones," by Max Bauer, translated by L. J. Spencer, M.A. "Mineralogy," by H. A. Miers, D.Sc., M.A., F.R.S. "A Text Book of Mineralogy," by E. S. Dana.
Miscellaneous.
Famous Diamonds of the World.
Some Famous Diamonds.
Birth Month Stones According to Various Nations.
Gems for the Days of the Week.
The proper stones for rings to be worn on certain days of the week are as follows:
Gems and Their Significance.
Significance of Gems.
Birthday Flowers, Zodiacal Signs of Flowers and Wedding Anniversaries.
Flowers.
The following shows five different lists of the flowers of the month, taken from various authorities: